Abstract
Introduction Allogeneic hematopoietic cell transplantation (HCT) is the treatment of choice for many inborn errors of immunity (IEI). Impaired recovery of thymic function predicts poor clinical outcomes after HCT, especially in pediatric patients with IEI. CD31+CD4+CD45RA+ T cells are recognized as recent thymic emigrants (RTE) or true naïve T cells, and indicators of thymic output in HCT recipients. Reduced intensity-conditioning (RIC) and reduced toxicity-conditioning (RTC) regimens are increasingly used to limit organ toxicity and late effects of HCT. However, it is not known if they facilitate thymic recovery. Furthermore, graft-versus-host disease (GVHD) and its treatment can lead to prolonged thymic dysfunction.
Methods Pediatric normal values and percentiles for a flow cytometry based RTE assay were developed. In this prospective study, we used RIC with fludarabine, melphalan, alemtuzumab, RTC with busulfan, fludarabine, alemtuzumab or myeloablative conditioning regimen (MAC) with busulfan, cyclophosphamide, alemtuzumab in patients with IEI. For GVHD prophylaxis cyclosporine A or tacrolimus together with mycophenolate mofetil were used. We assessed RTE immune reconstitution at 100 days, 6 months, 1 year and 2 years after HCT. Institutional review board of the University of Minnesota approved the study protocol. We hypothesized that thymic-derived immune reconstitution is negatively affected by occurrence of acute GvHD, age at transplantation (negatively with higher age) and type of conditioning (negatively with more intensive conditioning). Linear mixed model was performed for the repeated measures of RTE (after square root transformation) with covariates time (discrete), age at transplant (continuous), conditioning regimen, acute GHVD and all the second-order interactions with time.
Results Between January 1st 2012 and December 31st 2021 45 patients with IEI were transplanted. Two patients did not receive the conditioning regimen per protocol, one patient received alemtuzumab only and two patients received no conditioning. These five patients were excluded from the final analysis. Thirteen (32%) of 40 patients were female. Median age at transplantation was 2.64 years (IQR 0.89 to 10.09). Median time to neutrophil engraftment was 12 days (IQR 10 to 14). Median follow-up time was 46.5 months (IQR 22.8 to 62.7). The overall survival was 82.5% (95%CI 66.8% to 91.2%). 13 (32%) received RIC, 23 (57%) RTC and 4 (10%) MAC. 10 (25%) patients had a matched related donor, 24 (60%) had a matched unrelated donor and 6 (15%) received an umbilical cord blood transplant. 7 (17.5%) patients had Chronic Granulomatous Disease, 5 (12.5%) Hemophagocytic lymphohistiocytosis, 5 (12.5%) Severe congenital neutropenia, 4 (10.0%) CD40 Ligand deficiency, 4 (10.0%) Severe combined immunodeficiency, 4 (10.0%) immunodeficiency not otherwise specified, 3 (7.5%) Wiskott-Aldrich syndrome, and 8 (20%) other primary immunodeficiency as underlying diagnosis.
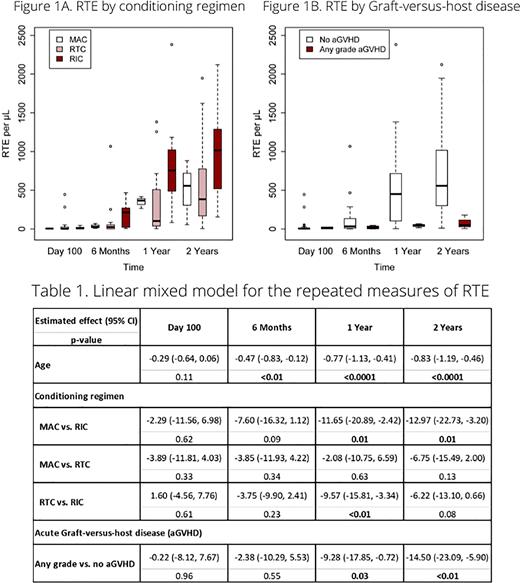
Patients with more intensive conditioning had lower RTE absolute values at one- and two-years post-transplant than patients with less intensive conditioning regimen (Figure 1A). Patients with acute GHVD occurring in the first 100 days after transplantation had lower RTE absolute values at one- and two-years post-transplant compared to patients without GVHD (Figure 1B).
In the linear mixed model for the repeated measures of RTE (after square root transformation), we found at one-year post-transplantation a significant difference with increasing age (p<0.0001), between any grade acute GVHD and no acute GVHD (p=0.03) and between MAC and RIC (p=0.01) and RTC and RIC (p<0.01) (Table 1). These results remained robust at two-year follow-up as well.
Conclusions Serial measurement of RTE is an useful immune reconstitution marker for assessment of thymic function after HCT. In patients with IEI, age at transplantation, occurrence of acute GHVD and intensity of conditioning regimen were strongly associated with slower thymic-derived immune reconstitution.
Disclosures
Dong:Quest diagnostics: Current Employment. Abraham:Beckman Coulter: Honoraria; Enzyvant: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.